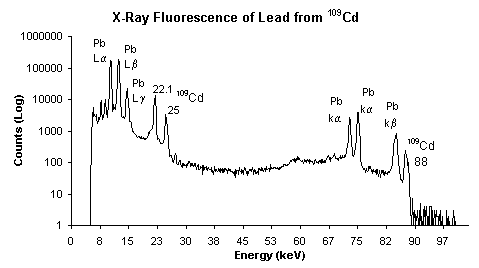
Spectrum taken using Amptek XR-100CR 5mmX5mmX500µm X-Ray Detector (20µs shaping time - optional feature) and Amptek MCA8000A Multichannel Analyzer.
 ®
®
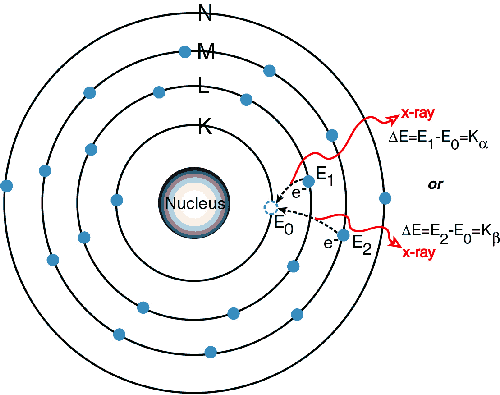
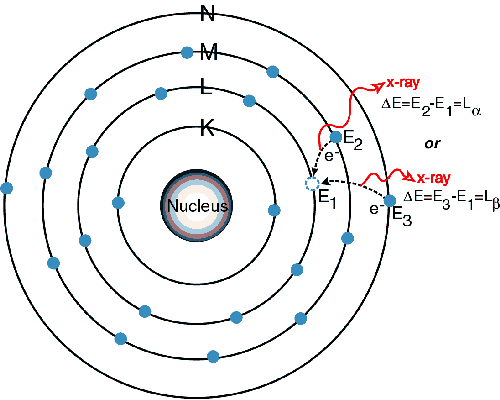
When a primary x-ray excitation source from an x-ray tube or a radioactive source strikes a sample, the x-ray can either be absorbed by the atom or scattered through the material. The process in which an x-ray is absorbed by the atom by transferring all of its energy to an innermost electron is called the "photoelectric effect." During this process, if the primary x-ray had sufficient energy, electrons are ejected from the inner shells, creating vacancies. These vacancies present an unstable condition for the atom. As the atom returns to its stable condition, electrons from the outer shells are transferred to the inner shells and in the process give off a characteristic x-ray whose energy is the difference between the two binding energies of the corresponding shells. Because each element has a unique set of energy levels, each element produces x-rays at a unique set of energies, allowing one to non-destructively measure the elemental composition of a sample. The process of emissions of characteristic x-rays is called "X-ray Fluorescence," or XRF. Analysis using x-ray fluorescence is called "X-ray Fluorescence Spectroscopy." In most cases the innermost K and L shells are involved in XRF detection. A typical x-ray spectrum from an irradiated sample will display multiple peaks of different intensities.

Spectrum taken using Amptek XR-100CR 5mmX5mmX500µm X-Ray Detector (20µs shaping time - optional feature) and Amptek MCA8000A Multichannel Analyzer.
The characteristic x-rays are labeled as K, L, M or N to denote the shells they originated from. Another designation alpha (a), beta (b) or gamma (g) is made to mark the x-rays that originated from the transitions of electrons from higher shells. Hence, a Ka x-ray is produced from a transition of an electron from the L to the K shell, and a Kb x-ray is produced from a transition of an electron from the M to a K shell, etc. Since within the shells there are multiple orbits of higher and lower binding energy electrons, a further designation is made as a1, a2 or b1, b2, etc. to denote transitions of electrons from these orbits into the same lower shell.
The XRF method is widely used to measure the elemental composition of materials. Since this method is fast and non-destructive to the sample, it is the method of choice for field applications and industrial production for control of materials. Depending on the application, XRF can be produced by using not only x-rays but also other primary excitation sources like alpha particles, protons or high energy electron beams.
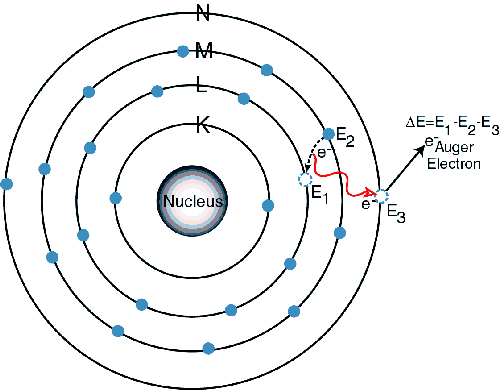
Sometimes, as the atom returns to its stable condition, instead of emitting a characteristic x-ray it transfers the excitation energy directly to one of the outer electrons, causing it to be ejected from the atom. The ejected electron is called an "Auger" electron. This process is a competing process to XRF. Auger electrons are more probable in the low Z elements than in the high Z elements.
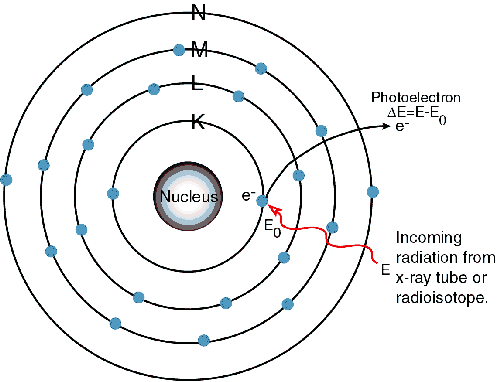
1) An electron in the K shell is ejected from the atom by an external primary excitation x-ray, creating a vacancy.
X-Ray Fluorescence Spectroscopy in PDF Format (56k).
Revised February 5, 2001